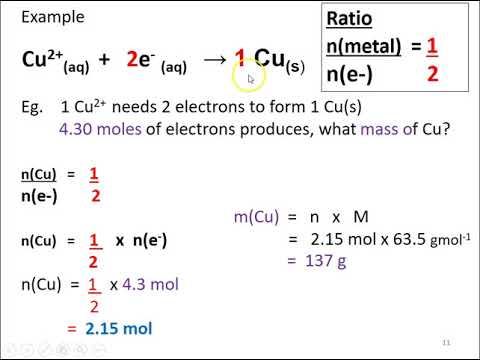
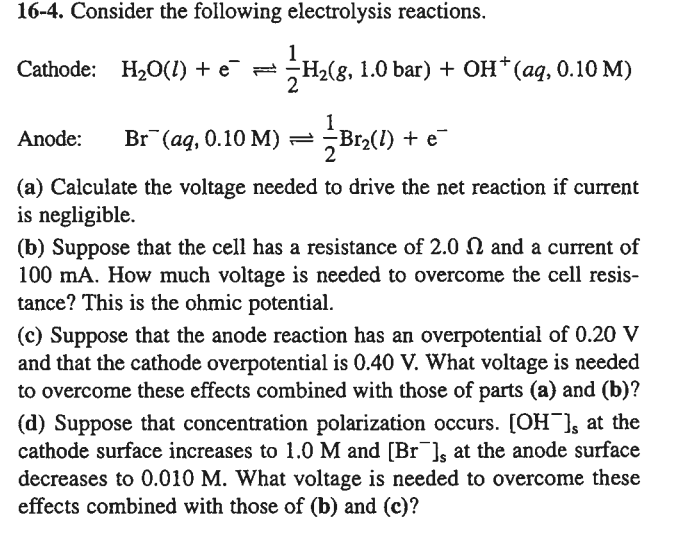
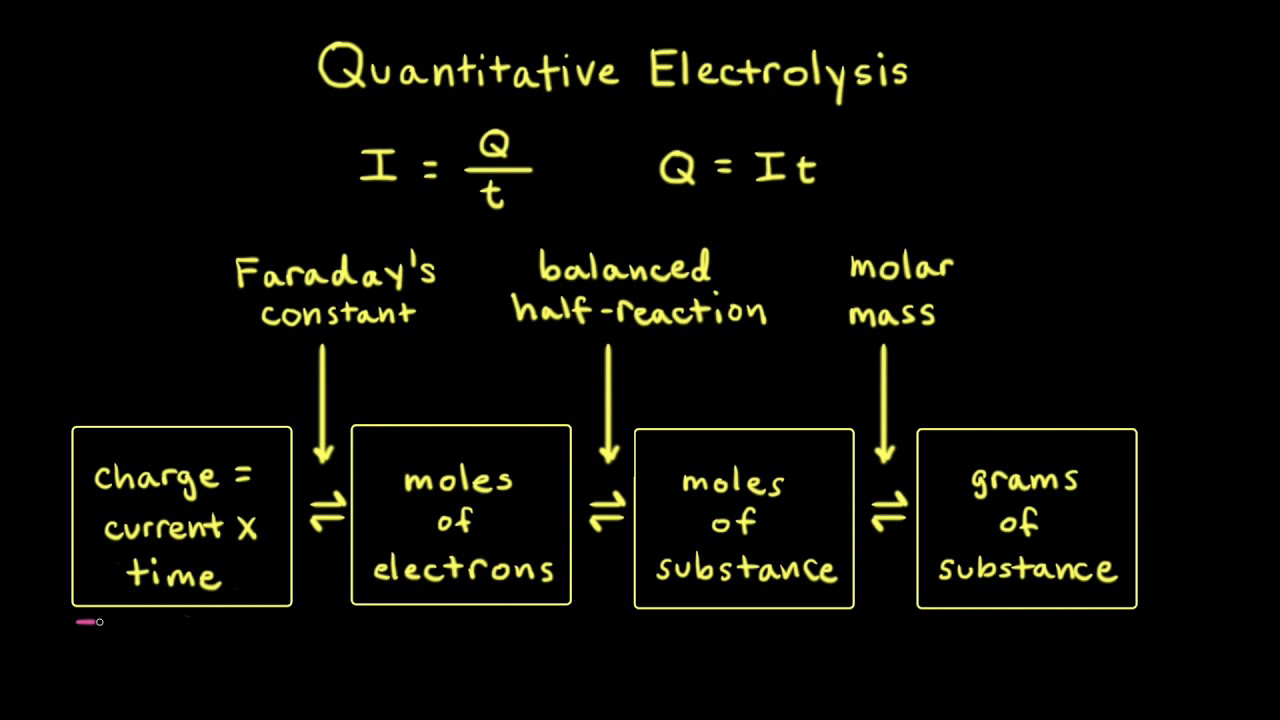
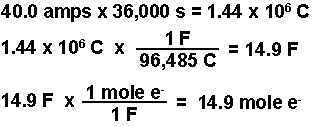SOLVED: Electrolysis of aqueous solution of 0.1 M CuBrz was carried out using inert electrode at 25 Determine the products of electrolysis at anode and cathode: Justify. Calculate the mass of product
Unit 3 PPA 2 QUANTITATIVE ELECTROLYSIS. QUANTITATIVE ELECTROLYSIS (Unit 3 PPA 2) The aim of this experiment is to determine the quantity of electricity. - ppt download
Welcome to Chem Zipper.com......: During electro refining of Cu how much time is needed to produce 250g Cu on the cathode if the current is kept at 11 A?
20. Calculate the quantity of electricity in coulomb which liberates enough hydrogen at the cathode during electrolysis of acidified water so that it can fill a ballon of capacity 10 litres at