The weight percent of source (formula weight = 342 g mol^-1 ) in an aqueous solution is 3.42. The density of the solution is 1 g mL^-1 , the concentration of sucrose
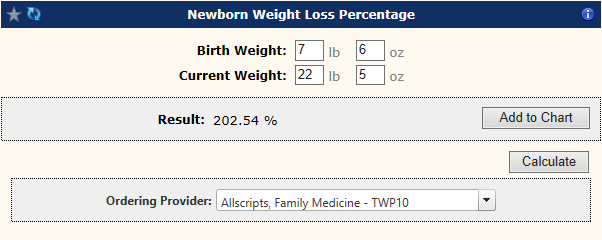
Galen eCalcs - Calculator: Newborn Weight Loss Percentage - Galen Healthcare Solutions - Allscripts TouchWorks EHR Wiki
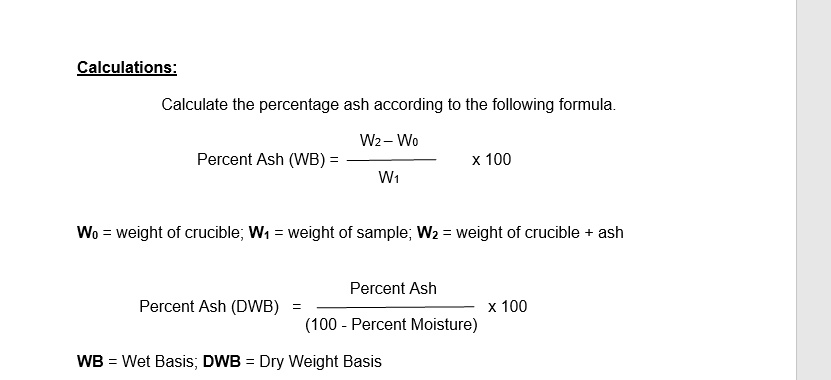
SOLVED: Calculations: Calculate the percentage ash according to the following formula: Wz Wo Percent Ash (WB) X 100 W1 Wo weight of crucible; Wt weight of sample; Wz weight of crucible ash

Percent Concentration Calculation- (Part-02) Weight by Weight (W/W) With Easy explanation (HINDI) - YouTube

Step-by-step guide to the derivation of percentage study weights in... | Download Scientific Diagram
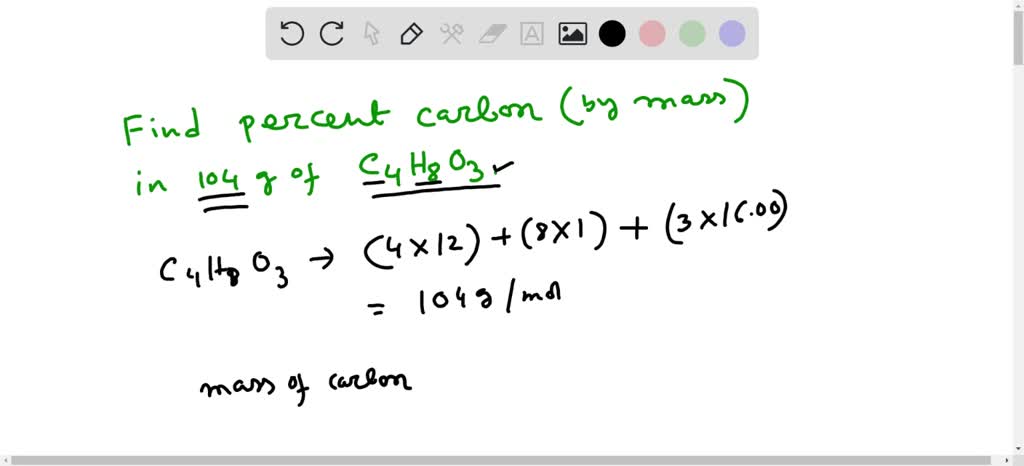
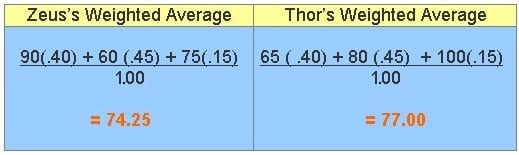
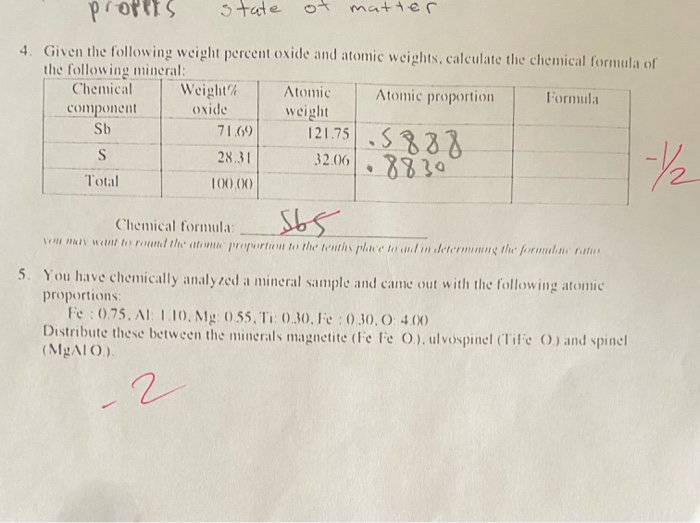
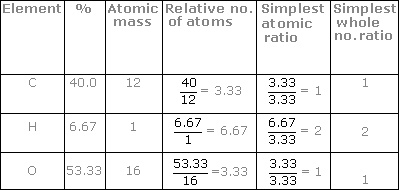

:max_bytes(150000):strip_icc()/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)